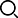Development Process
Development Process
2025/06
The company's product "Sunitinib Malate Capsules" was approved for listing.
2025/03
The company's product "Imatinib Mesylate Tablets" was approved for listing.
2024/12
The company's product "Erlotinib Hydrochloride Tablets" was approved for listing.
2024/11
The company's product "lenalidomide" was approved for listing.
2024/09
The company changed its name to “Guangzhou Keruite Pharmaceutical Co., Ltd.
2023/06
Acquisition of a pharmaceutical manufacturing license
2022/01
The pharmaceutical production license obtained
2020/05
Pharmaceutical factory put into use
2018/01
Construction of pharmaceutical factory started
2017/07
Set up research and development center, supporting domestic and foreign advanced research and development and testing equipment
2013/06
Company incorporation
Address: No.80, Spring Equinox Road, Huangpu District, Guangzhou
Telephone:020-8328 9091
Adverse Drug Reaction/Event Feedback E-mail:pvreport@bio-current.com