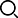The raw material manufacturing workshop contains the following facilities:
●It has 4 commercial GMP multi-functional production lines (including the special line of tenib and general medicine), all equipped with automatic temperature control system (such as DCS and TCU system), and the overall production capacity can reach 50t-75t/year.
●The common synthesis area production line is equipped with 41 glass-lined/stainless steel reactors ranging from 100L to 5000L (Confirm the temperature: -20°C ~ 130°C), PSB600~PSB1200 stainless steel and 15 centrifuges lined with halar, as well as matching drying equipment and filtering equipment.
●The clean area is equipped with 500L, 2000L and 3000L stainless steel reaction kettles.
●The clean area is equipped with imported and domestic micropowder equipment, which can be crushed to a particle size of less than 10μm.
●The production base has complete facilities for API production and a perfect quality management system has been established.


Custom Services
1. Process Transfer
● Process route optimization/enlargement production/granularity exploration, etc.
● Continuous cost optimization
● Process safety risk assessment
● Output of supporting documents for process technology transfer
2. Process Validation and Registration Declaration
● Intermediate storage time limit validation/cleaning effect validation
● Production and regulatory coordination of process validation batches
● Writing of the application documents
● Regulatory department consultation and communication
3. Analysis and Testing
● Stability study
● CMC registration documentation support
● Development, validation and validation of analytical methods
4. API Production
● Equipped with TCU and DCS automation system, automatic control of precise temperature control, remote material transportation, remote equipment start and stop, and alarm prompts are configured to achieve stable and safe production.
● Have experienced technical management personnel and production personnel to stabilize production and ensure the supply of APIs.
● Adhere to the values of honesty and trustworthiness, and adhere to the compliance and standardized operation.
Laboratory Overview
At present, the laboratory is equipped with a batch of precision testing instruments and equipments,such as 27 liquid chromatographs, 4 gas chromatographs, 5 Wahyong dissolution instruments, Shimadzu infrared spectrometer, Shimadzu atomic absorption spectrophotometer, Shimadzu ultraviolet spectrophotometer, Bettersize Laser Particle Size Analyzer , OMEC Laser Particle Size Analyzer, German NETZSCH DSC and eight stability test boxes .They can meet the requirements of testing and stability test of various materials,APIs and preparation products. At the same time, the Thermo chameleon network chromatographic system, the Waters Empower 3 network chromatographic system, and the Thermo LIMS laboratory information management system were introduced to achieve the information management of inspection data and inspection process and ensure the reliability of inspection data.
The laboratory has successfully completed method transfer for more than 11 API products and methodological validation for more than 230 test items. We have completed the method transfer of 8 tablets and capsules, accumulated rich product transfer experience, and can provide strong technical support for project implementation.

Comprehensive Quality Management System
We have professional quality management personnel who have the quality management capability from R&D to product launch.

Efficient Transformation Team
Implement project-based management to ensure smooth process from transfer to industrialization and strict control measures.

Perfect Hardware Facilities
Laboratory inspection instruments are available, including gas phase liquid chromatograph, ultra-high performance liquid chromatograph, infrared spectrometer and other advanced equipment.

Rich Registration and Inspection Experience
Each year, 3 or 4 varieties have been successfully submitted for registration and a number of supplementary studies have been completed.